| T O P I C R E V I E W |
| jlp1528 |
Posted - 05/23/2020 : 07:59:29
So here's the story. I've had the GMC-500+ for a few months now, and I've known for quite a while that anything with potassium in it contains potassium 40 and is thus slightly radioactive. I'm not sure why, but the banana is the classic example.
Considering this, I was under the impression that potassium permanganate, chemical formula KMnO#8324;, would contain enough potassium to be detectable with a Geiger counter. Unfortunately, the GMC-500+ CPM wouldn't budge from background. At this point I just figured I was wrong - that there wasn't enough potassium in this particular compound to matter - and moved on.
Here's where things get weird. For entirely unrelated reasons, I finally bit the bullet (money wise) and sprang for the GMC-600+ last week. Of course, this model is actually quite a reasonably priced detector for this level, but $300 is still $300. Anyway, this model *does* show an elevated reading on the potassium permanganate - over 200 CPM vs a background of about 40.
This is puzzling though, since the energy levels of potassium 40 decays are well over 1 MeV - several times higher than the stated minimums of 250 KeV beta and 100 KeV gamma in the GMC-500+ manual. So shouldn't both the 500+ and 600+ be able to read this stuff? Sure, the tube in the 500+ is both somewhat larger and much more sensitive, but if the decay energy is above the detection threshold of both, that shouldn't matter in terms of defecting the sample at all, right? What am I missing here...? |
| 9 L A T E S T R E P L I E S (Newest First) |
| ullix |
Posted - 05/03/2021 : 01:00:14
quote:
Potassium beta is quite weak ...
... you will need a lot of potassium to detect the radiation.
No and No!
K40 decays with both beta and gamma. The relative proportions are 90% beta, and 10% gamma. The gamma is 1.46 MeV, which is a rather high energy among all naturally occurring gammas. Beta has a max energy of 1.33 MeV, and it also is on the high side of beta energies.
With the high gamma energy, the chance of absorption is on the low side, though it clearly can be detected in a Geiger counter.
The problem with betas - all betas, no matter what the source - is, that the specified energy is only the maximum of beta energies from this source. There is a continuum of lower energies down to zero. See a typical spectrum in next pic:
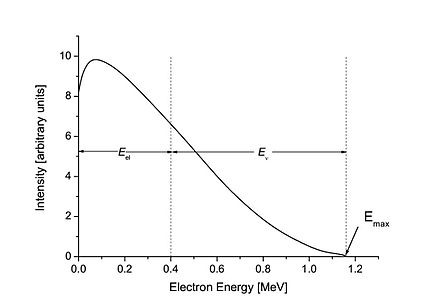
Betas from all energies, be they high or low, will be caught by the the tube. That is, if they reach the tube, and are not absorbed beforehand by the case of the counter, or even the air between counter and source, or any other material. Thus, if you really want to measure betas, remove the case of the counter, and bring the K-40 source close to the tube.
If you want to measure gammas only, make sure to put a proper absorber between counter and source.
This and more on K-40 measurements with even a simple GMC-300E counter can be found in my "Potty Training" and "Going Banana" articles.
https://sourceforge.net/projects/geigerlog/files/Articles/GeigerLog-Potty%20Training%20for%20Your%20Geiger%20Counter-v1.0.pdf/download
https://sourceforge.net/projects/geigerlog/files/Articles/GeigerLog-Going%20Banana.log.zip/download
You'll be surprised what one can do with K40!
|
| Searinox |
Posted - 05/02/2021 : 04:12:02
Potassium beta is quite weak and has trouble getting through the plastic back of the case as well as the glass wall of the tube. I have a small fist-sized pack of it and my GMC-320+ struggles to read 0.20 uSv/h and it's more of statistical significance of how often it goes over that value versus normal background, than anything steady. By contrast my GMC-600 makes it immediately clear that there are more clicks than usual and settles around 0.3-0.36 uSv/h. If you don't have a mica window, you will need a lot of potassium to detect the radiation. |
| kotarak |
Posted - 06/11/2020 : 15:16:12
quote:
Originally posted by jlp1528
Thank you for the information. After failing to trigger the GMC-500+ with KCl ("No Salt") I decided to try yet another potassium compound, another one I may actually use at that: KOH, aka caustic potash, aka put this in water to make Drano. The increase in CPM is minimal and slow, but it is definitely there. :)
If you are in US, just go to Home Depot or Lowes and look for the
"Water Softener" section. The regular softener is NaCl but they also sell KCl softener in 40 LB bags. If you go with your counter and they are well stocked, you'll have the chance to measure the activity of hundreds of pounds of KCl. |
| jlp1528 |
Posted - 06/11/2020 : 13:42:01
Thank you for the information. After failing to trigger the GMC-500+ with KCl ("No Salt") I decided to try yet another potassium compound, another one I may actually use at that: KOH, aka caustic potash, aka put this in water to make Drano. The increase in CPM is minimal and slow, but it is definitely there. :) |
| Damien68 |
Posted - 05/25/2020 : 01:28:19
a larger tube gives better observation and allow measurements to be made more quickly in proportion of his size, but will not fundamentally change the result.
A pancake is better for sample test because it is very directional, but for an ambient measurement the traditional tube is better.
something else:
if you are comfortable with math you can look at the following article:
https://en.wikipedia.org/wiki/Solid_angle
It is important to know that for geometric reasons, the number of particles detected (intercepted by the tube) is a function of the square of the distance between the source and the tube (following the solid angle value formed by the active surface of the tube, and a center located at the source location).
This is largely why as soon as we remove the counter from the source, the detection decreases quickly.
There is also the fact that not all particles go very far.
Simulation bellow show a 10x10x10mm sample source with a SBM-20-1 and a LND7317 GM tube both at 10mm from the source.
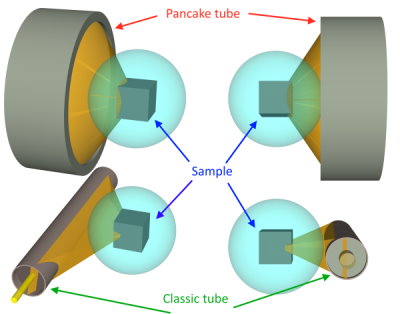
we can see that in the case of the classic tube, the brown / orange cone is much narrower in the direction of its projection with respect to the thickness of the tube. in reality, it should even be a little narrower than that because of the tangent effects.
|
| ullix |
Posted - 05/25/2020 : 00:23:19
If you wonder about Potassium, it is time for some "Potty Training"!
See my article on this subject here: https://sourceforge.net/projects/geigerlog/files/Articles/GeigerLog-Potty%20Training%20for%20Your%20Geiger%20Counter-v1.0.pdf/download
And if you are wondering about Going Banana with Potassium, I also show how this can be done: https://sourceforge.net/projects/geigerlog/files/Articles/GeigerLog-Going%20Banana-v1.0.pdf/download
And all of this done with a plain old GMQ 300E+ and my program GeigerLog, available on the same site.
You just need a bit of patience; getting the statistic good enough takes time.
|
| jlp1528 |
Posted - 05/23/2020 : 16:31:11
I did wonder if the simple increase in size could be a factor. Good points on this and my other post that the energy level specs might not be accurate. Thanks for the detailed info and examples! Still learning here; I'll stop learning when I'm dead. Maybe. ;) |
| Damien68 |
Posted - 05/23/2020 : 13:06:55
We often talk about bananas because it is one of the fruits that contains the most potassium.
Pancake tube like one present in GMC-600+ was originally made to test samples and not specifically to test alpha radiation.
From sample, Each particle is emitted in a totally random direction. We should talk about solid angle and integration... but it will become complex. To simplify, we will say that the pankake tube will cover half of the surface around the sample to be tested, that is to say that if a particle is emitted by the potasium, it has One chance out of two to crossing the pankake tube and to be tetected, and an other chance going to the other side and to not be detected.
For traditional tubes for example the M4011 present in the GMC-500 + the active surface of the tube around the sample will be much weaker, also the probability that a particle emitted by potassium is intercepted by the tube is much lower, in proportion to the covered (by the tube)/uncovered ratio of sphere surface around the sample.
But the pancake tubes also have their shortcomings (directional, size, saturation ...).
Otherwise I did a quick calculation:
The activity of 1 g of pure K40 is 263 kBq, This means that one gram of pure K40 will emit 263 thousand particles per second.
On the other hand: 1g of standard potasium on earth contains 0.012% of K40.
So activity of 1 g of standard potasium is 263k x 0.012/100 = 31 Bq, This means that one gram of standard potasium will emit 31 particles per second.
for example I bought a 240 g box of diet salt composed of 40% potassium.
It must therefore emit 240 x 0.4 x 31 = 2976 particles per second.
I emptied the salt shaker in a ziploc bag, then I put my 500+ on it, I then have an elevation of only 60 CPM and I speak well of CPM.
In theory we should have 2976 * 60 = 178560 particle emited per minutes, but most of it must not leave the Ziplock, certainly it must be intercepted by other nucleus or atomes, and of all the particles that come out of my bag, the tube of my 500+ will detect only 0.03%
|
| kotarak |
Posted - 05/23/2020 : 09:54:08
For what is worth, I just measured a 20Lb. bag of Potassium Chloride with my 600+ and I get around 470-500 CPM (my background is 42-45CPM).
I think, what you are seeing has to do with the sensitivity of the M4011 tube in 500+. This is a Chinese glass tube, somewhat comparable to the Russian SBM-20 in sensitivity.
On the other hand 600+ is using a US made and way more expensive LND 7317 which is practically the low-voltage version of LND 7311 - used by Ludlum model 3 and other high-quality counters).
I think it might be that the specs of M4011 are loose and the actual sensitivity to low-energy beta is just not comparable to the LND tubes (with a mica window, friendly to alpha particles but I am sure beta sensitivity doesn't suffer too) |
|
|

